Acid-base balance and tissue acidosis
For the health of our bones, muscles, cartilage, and tendons, we need a good acid-base balance in the body
Let's see what it consists of:
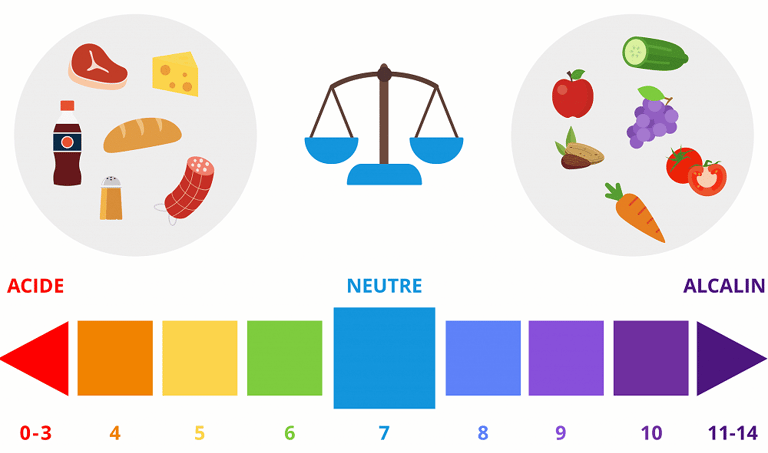
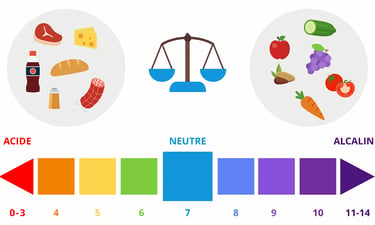
What we call the "pH" of something is its hydrogen potential, i.e., a measure of its H+ ion concentration. A pH of 7 is neutral, while a pH below 7 is acidic and a pH above 7 is basic (or alkaline). During the day, with our physical activity and diet, our body becomes more acidic. During the night, with rest and the absence of food, it becomes alkaline, and the balance is thus maintained.
A frequent problem, particularly affecting athletes (who lose a lot of alkalizing minerals through perspiration and urine), is excess acidity in the body or "tissue acidosis". This problem also affects heavy consumers of soft drinks, which are rich in phosphorus. The body then draws calcium, which is alkaline, from the bones to counteract the acidic load, leading to bone demineralization. Other negative consequences affect muscles and joints.
If tissue acidosis is suspected, it is useful to perform a urine pH test at different times of the day, as the body seeks to rid itself of excess acids through the urine. Test strips are available from chemists. I recommend taking three measurements a day for five days: on the second morning urine, the one before lunch and the one before dinner. The normal pH of urine for a healthy person with a balanced diet is 6.5 to 7.5. If the average of the three measurements is below 6.5 over five days, it's reasonable to conclude that tissue acidosis is present.
In addition to an excessively acidic diet, other possible causes include poor breathing, when you don't use your full lung capacity, resulting in reduced pulmonary elimination of volatile acids. There are also medical causes, such as kidney or liver disease. Excessive sugars and fats also cause acidification, by fermentation of glucose or production of ketone bodies. Salt consumption is also acidifying.
Tissue acidosis can be remedied by treating the cause. For example, if the cause is respiratory, you need to take care to breathe properly and stop smoking if you're a smoker. If the cause is dietary, you need to correct your diet, notably by eating far more vegetables, which are alkalinizing thanks to the minerals they contain, and by reducing your consumption of meat and cheese, which are acidifying, as well as avoiding excess sugar, fat and salt. Vegetables and fruit are alkalinizing, cereals and legumes are slightly acidic, meat and fish are acidic, and cheese is very acidic. For a more refined approach, you can consult the PRAL ("potential renal acid load") indices of foods on the Internet: the higher the positive index, the more acidic the food; the more negative the index, the more alkaline the food:
Yuka, Le guide de l'alimentation saine, published by Éditions Marabout and available at https://yuka.io/
Counter-intuitively, citrus fruits and apples, which taste acidic, are in principle alkalinizing, as digestion neutralizes their acids. Two exceptions, however: this is not the case for people already suffering from acidosis (so avoid apples and citrus fruits when trying to correct acidosis, until it has been cured), nor, it seems, for long-boned people with thin bones (exact causes unknown).
If necessary, you can speed up the deacidification process by supplementing with citrates of calcium, magnesium, potassium, and silicon.
To find out more, also read these articles:
About nutritional risks for athletes: https://isabellemaesnutrition.com/en/athletes
Balance scale drawing by www.compagnie-des-sens.fr